Computerized system validation(CSV)
Time:2019-05-20
Our company has rich experience in the implementation of computerized system validation in GMP environment, and the validation method has been widely used in the pharmaceutical industry.
The CSV uses a lifecycle based GAMP5 V model to define the required validation documents and responsibilities for each stage. We can also assist customers to reasonably define user needs (URS) and assist customers to conduct professional risk analysis (RA).
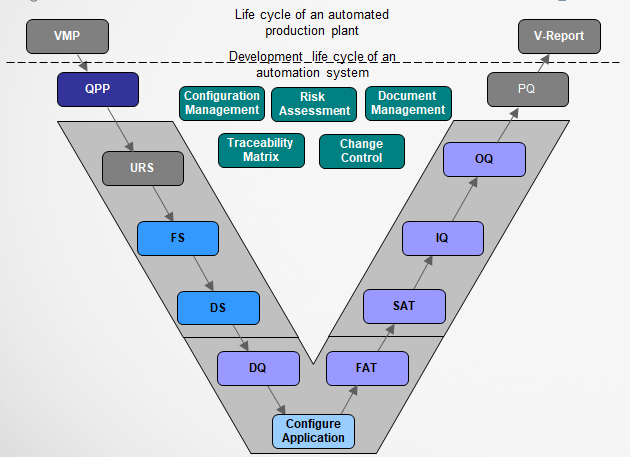 VMP - Validation Master Plan
QPP - Quality & Project Plan
URS - User Requirement Specification
SOP- Standard Operation Procedure
FS - Function Specification
DS - Design Specification
DQ - Design Qualification
FAT – Factory Acceptance Test
SAT – Site Acceptance Test
IQ – Installation Qualification
OQ – Operational Qualification
PQ – Performance Qualification
V-Report – Validation Report
VMP - Validation Master Plan
QPP - Quality & Project Plan
URS - User Requirement Specification
SOP- Standard Operation Procedure
FS - Function Specification
DS - Design Specification
DQ - Design Qualification
FAT – Factory Acceptance Test
SAT – Site Acceptance Test
IQ – Installation Qualification
OQ – Operational Qualification
PQ – Performance Qualification
V-Report – Validation Report

